Developing Standardized Protocol for the Preparation of Caenorhabditis elegans Samples Suitable for Microfluidic Chip Loading
-
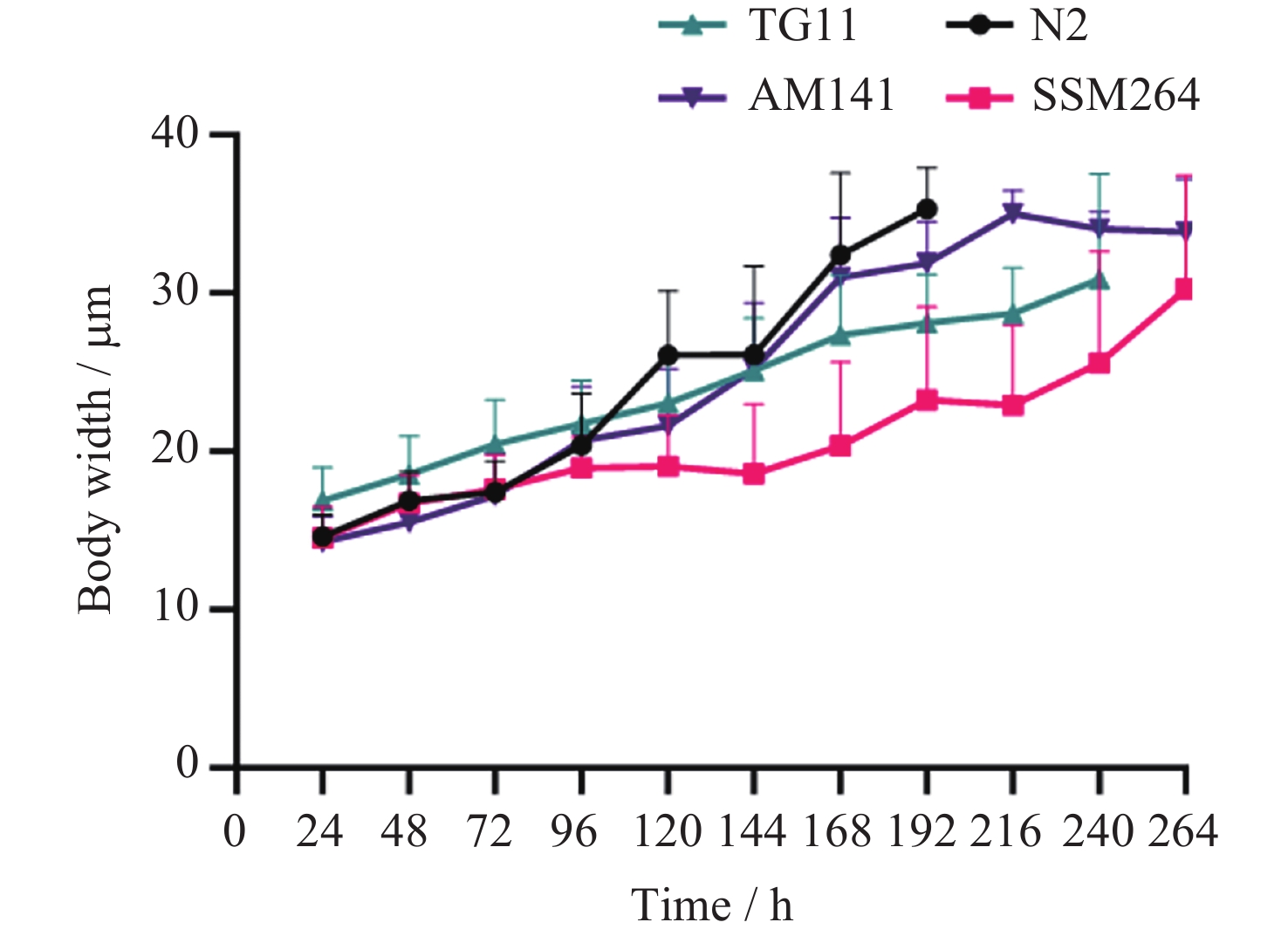
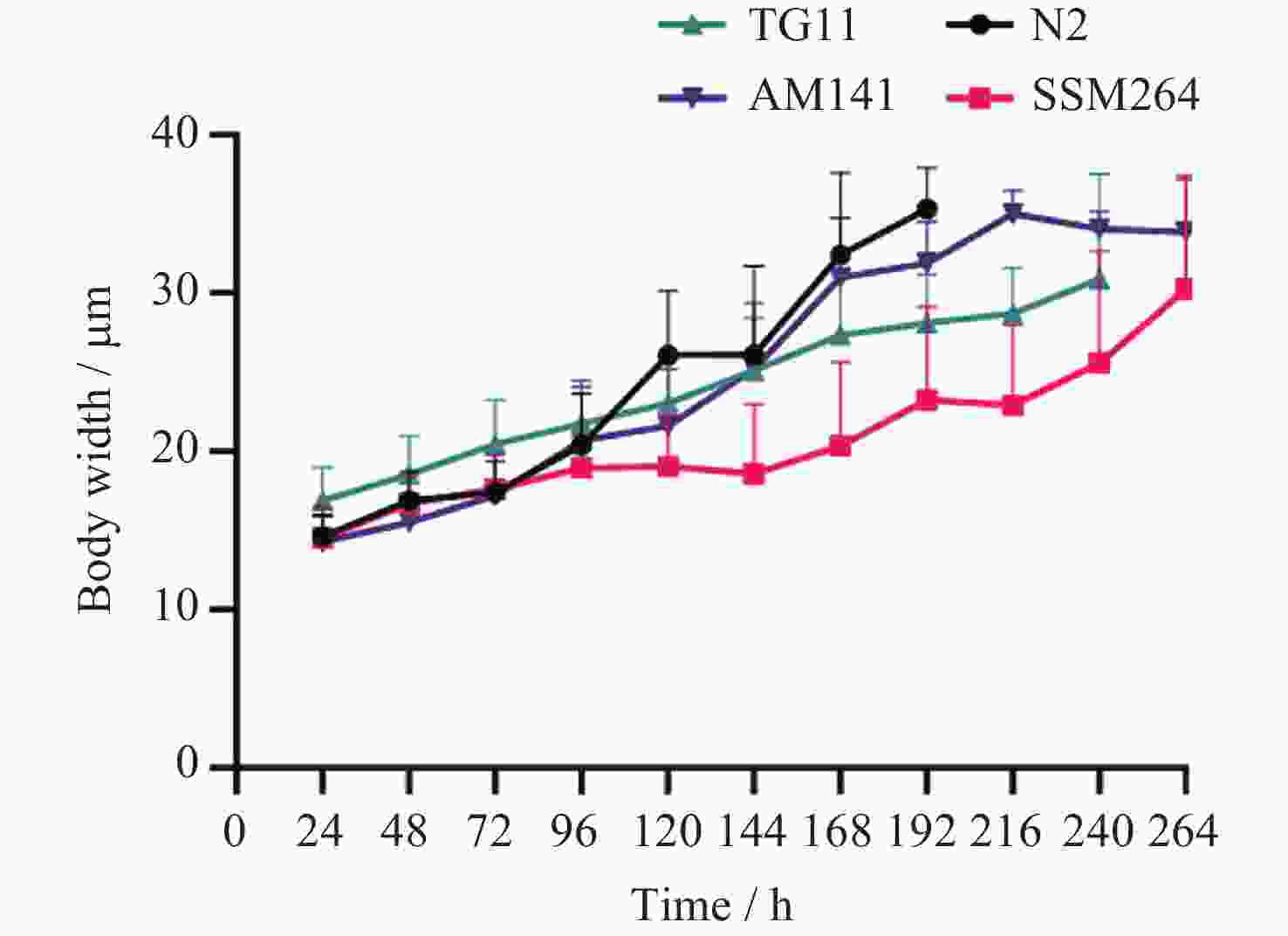
摘要: 随着中国空间站舱外辐射暴露平台的逐步应用, 空间辐射造成的长期生物损伤是空间生命科学亟待研究的方向. 秀丽隐杆线虫作为空间飞行实验的模式生物, 其辐射效应研究能够为人类深空辐射风险评估和防护提供重要依据. 为实现长期空间飞行下线虫的发育分析, 需依托微流控芯片液体培养系统进行搭载与观测. 为明确微流控芯片线虫样品的发育制备要求和加载方案, 研究采用灼烧法对野生型、DNA损伤修复蛋白(RAD-51, CEP-1)和肌肉运动蛋白(UNC-54)荧光品系在不同扩繁周期和发育时间进行群体体宽测量, 确定不同品系最适的扩繁和发育时长, 弥补芯片加载时样品同步性不足的问题. 利用上述流程制备的四种线虫加载于神舟十六飞船任务的线虫芯片, 样品体宽范围为27.71~28.02 μm, 符合芯片加载要求(24~29 μm), 保证了芯片内个体状态的一致性. 本研究建立了线虫扩繁-同步化-体宽控制-芯片加载的实验流程, 为空间站液体培养线虫搭载和观测提出“质控”要求.Abstract: As space biology experiments transition from short-term post-flight observations to long-term in-orbit monitoring, particularly with the application of the extravehicular radiation exposure platform on the Chinese Space Station, understanding the long-term biological effects of space radiation has emerged as an urgent research direction in space life sciences. Caenorhabditis elegans (C. elegans), which shares 60%~80% of homologous genes with humans, serves as a model organism for studying radiation effects in spaceflight experiments, providing essential insights for assessing and mitigating radiation risks in deep-space exploration. To enable long-term analysis of C. elegans development both inside and outside the spacecraft, a microfluidic chip-based liquid culture system can be utilized for single-individual worm loading and observation. Microfluidic chips regulate the number of nematodes entering the culture chamber by controlling the inner diameter of the channels. Therefore, the preparation and loading of chip samples impose precise requirements on the body width of nematodes, which is directly related to their developmental stage. To clarify the developmental requirements and loading protocols for nematode samples in microfluidic chips, this study measured the body width of wild-type (N2), DNA damage repair proteins (RAD-51, CEP-1), and muscle motility proteins (UNC-54) fluorescent strains of nematodes during different population proliferation cycles and larval development using the heat-shock method. This approach determined the optimal proliferation duration and developmental period for different nematode strains, addressing the issue of insufficient sample synchronization during microfluidic chip loading. After preparation using the aforementioned protocol, four types of nematodes were loaded onto the nematode chip for the Shenzhou 16 mission. The body width of the samples ranged from 27.71 μm to 28.02 μm, which meets the chip loading requirement of 24 μm to 29 μm and ensures consistency in the individual states within the chip. This finding validates the feasibility of the preparation protocol. This study established an experimental workflow for nematode “proliferation-synchronization-body width control-chip loading”, and proposed “quality control” requirements for nematode cultivation and observation in liquid culture systems aboard space stations.
-
Key words:
- Caenorhabditis elegans /
- Liquid sterile culture /
- Microfluidic /
- Development
-
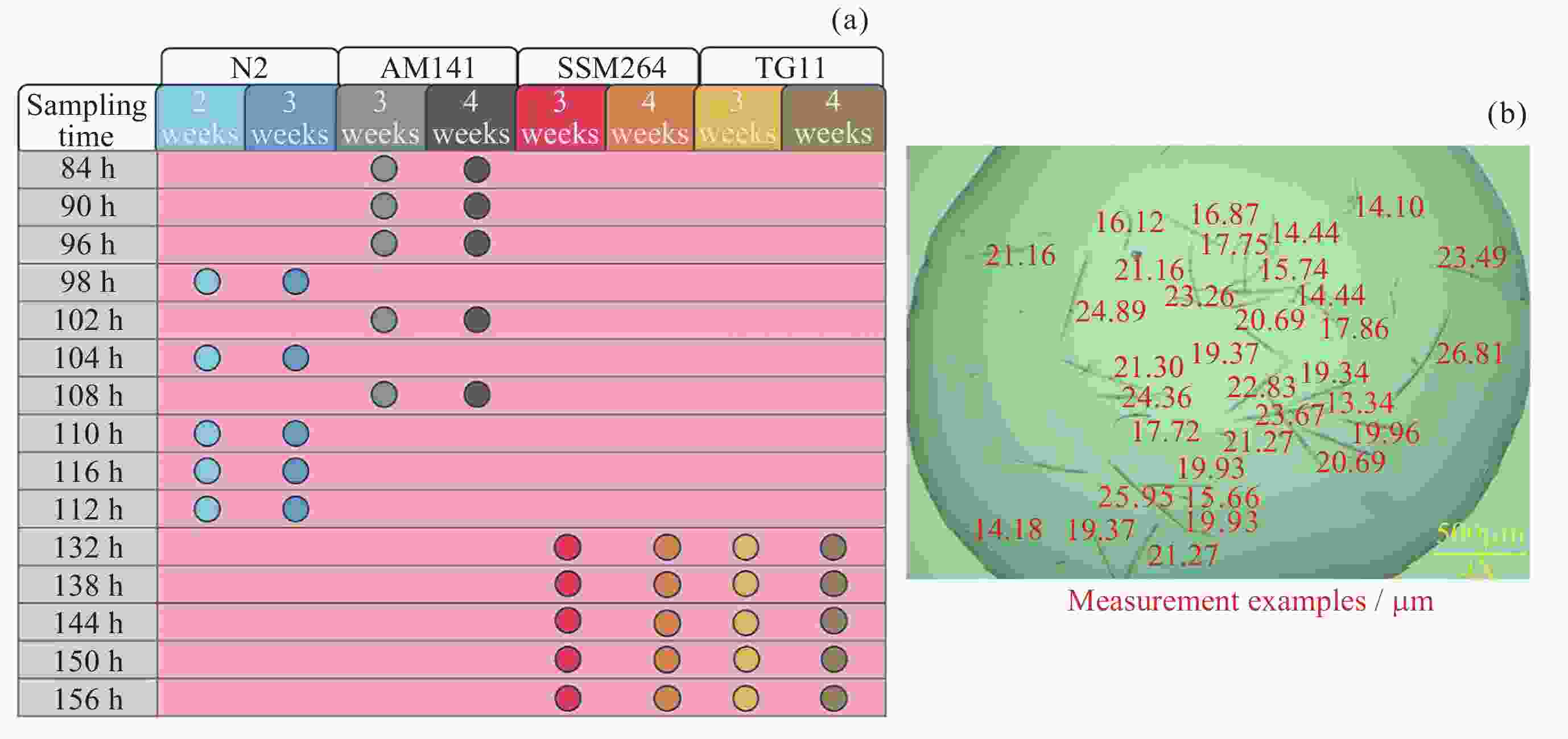
表 1 荧光标记线虫特征
Table 1. Fluorescently labeled nematode characteristics
线虫品系 TG11 SSM264 AM141 基因型 cep-1 (lg12501) I,
unc-119 (ed4) III, gtEx2rad-51 (iow53[GFP::rad-51]) IV/nT1[qIs51] (IV,V) rmIs133 [unc-54 p::Q40::YFP] 荧光标记
基因位点GFP绿色荧光标记cep-1 (DDR诱导的细胞凋亡检查点基因) GFP绿色荧光标记rad-51 (DBS引发HR修复招募蛋白基因,
编码RecA 重组酶)YFP黄色荧光标记unc-54 (肌球蛋白重链蛋白基因) 特点 L4期后20~40 h在生殖腺表达荧光, 咽泵也有荧光 群体中存在咽部荧光和非咽部
荧光的线虫, 需选择非咽部荧光的
rad-51纯合子在体壁肌细胞中显示绿色荧光, 多聚谷氨酰胺聚集 荧光位置 生殖腺荧光 咽部和生殖腺荧光 体壁荧光 表 2 N2线虫同步化后各发育时间体宽分布
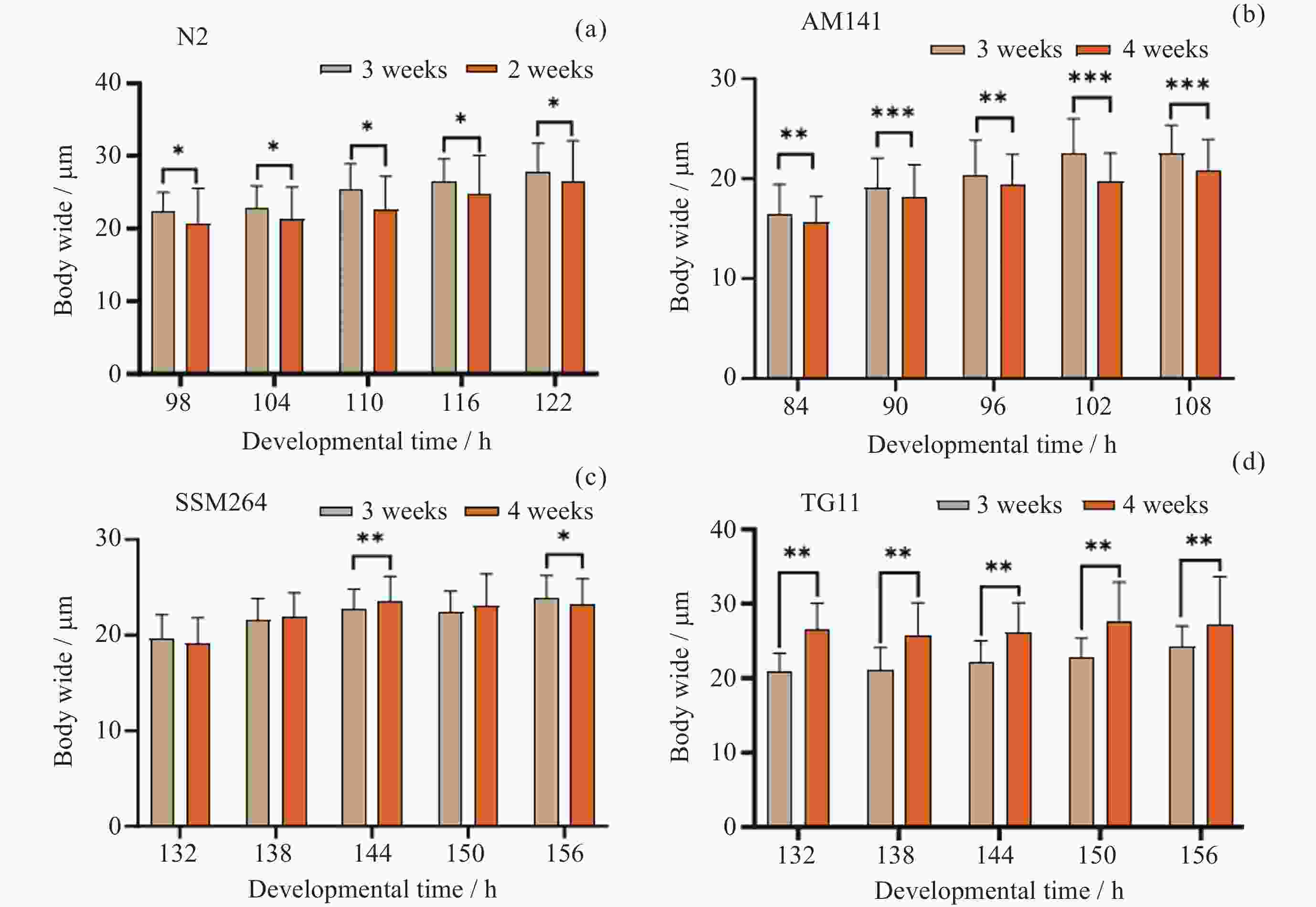
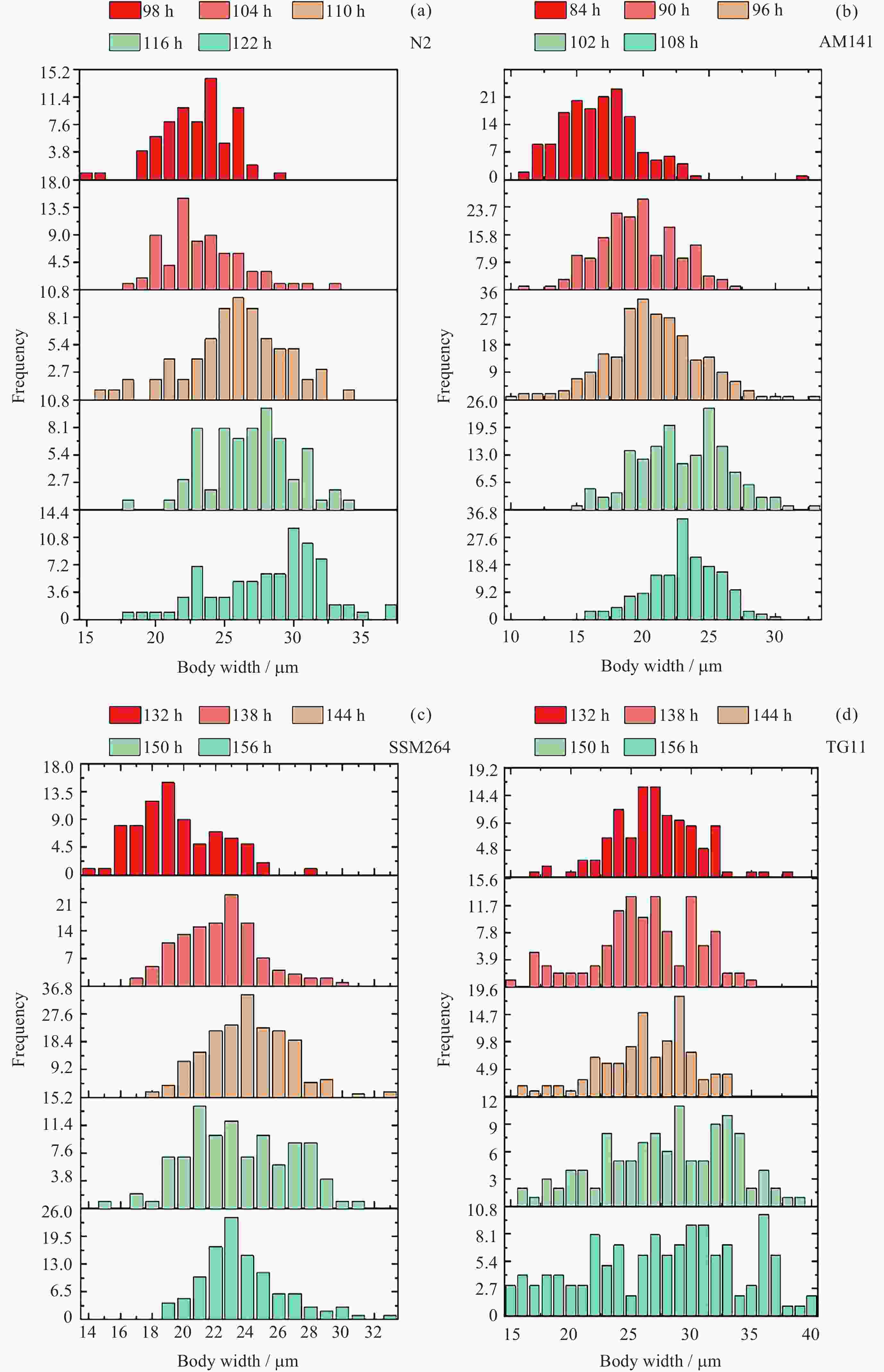
Table 2. Width distribution of N2 after synchronization
同步化后
发育时间/h均值/μm 24~29 μm
占比/(%)N24~29μm/
N>29μm98 22.42 25.71 $\infty $ 104 22.93 27.14 6.33 110 25.41 54.17 3.55 116 26.41 58.82 3.08 122 27.72 31.65 0.68 表 3 AM141线虫同步化后各发育时间体宽分布
Table 3. Width distribution of AM141 after synchronization
同步化后
发育时间/h均值/μm 24~29 μm
占比/(%)N24~29μm/
N>29μm84 16.36 0.00 $\infty $ 90 19.12 5.65 $\infty $ 96 20.26 8.28 $\infty $ 102 22.55 5.88 $\infty $ 108 22.57 17.02 40.00 表 4 SSM264线虫同步化后各发育时间体宽分布
Table 4. Width distribution of SSM264 after synchronization
同步化后
发育时间/h均值/μm 24~29 μm
占比/(%)N24~29μm/
N>29μm132 19.13 3.75 $\infty $ 138 21.84 15.00 18.00 144 23.51 39.27 25.00 150 23.05 37.62 19.00 156 23.20 25.93 5.60 表 5 TG11线虫同步化后各发育时间体宽分布
Table 5. Width distribution of TG11 after synchronization
同步化后
发育时间/h均值/μm 24~29 μm
占比/(%)N24~29μm/
N>29μm132 26.48 51.72 2.22 138 25.76 41.23 1.47 144 26.17 54.63 2.46 150 27.54 40.87 1.27 156 27.18 22.48 0.52 表 6 验证芯片加样时线虫群体状态
Table 6. Population status of the nematodes at the chip loading time
品种 发育
时间/h线虫
总数24~29 μm
线虫总数24~29 μm
线虫占比/
(%)N24~29μm/
N>29μmN2 104 93860 3677 3.91 $\infty $ AM141 96 51730 11639 22.50 $\infty $ SSM264 144 47997 19047 39.68 6.25 TG11 144 31465 7404 23.53 4.00 表 7 发射前线虫状态检验
Table 7. Nematodes status before launch
品种 平均体
宽/μm变异
系数/(%)线虫个数 存活率/
(%)N2 28.02 6.1 36 100 AM141 27.76 5.4 40 100 SSM264 27.85 5.2 33 100 TG11 27.71 5.4 35 100 -
[1] RUTTER L, BARKER R, BEZDAN D, et al. A new era for space life science: International Standards for Space Omics Processing[J]. Patterns, 2020, 1(9): 100148 doi: 10.1016/j.patter.2020.100148 [2] NIKONOROVA I A, DESRANLEAU E, JACOBS K C, et al. Polycystins recruit cargo to distinct ciliary extracellular vesicle subtypes in C. elegans[J]. Nature Communications, 2025, 16(1): 2899 doi: 10.1038/s41467-025-57512-3 [3] ZHU S H, ZHANG R S, YAO L X, et al. De novo NAD+ synthesis is ineffective for NAD+ supply in axenically cultured Caenorhabditis elegans[J]. Communications Biology, 2025, 8(1): 545 doi: 10.1038/s42003-025-07984-2 [4] QUACH K T, HUGHES G A, CHALASANI S H. Interdependence between SEB-3 receptor and NLP-49 peptides shifts across predator-induced defensive behavioral modes in Caenorhabditis elegans[J]. eLife, 2025, 13: RP98262 doi: 10.7554/eLife.98262 [5] MARKAKI M, TAVERNARAKIS N. Caenorhabditis elegans as a model system for human diseases[J]. Current Opinion in Biotechnology, 2020, 63: 118-125 doi: 10.1016/j.copbio.2019.12.011 [6] GARDEA E A, DENICOLA D, FREITAS S, et al. Long-term culture and monitoring of isolated Caenorhabditis elegans on solid media in multi-well devices[J]. Journal of Visualized Experiments, 2022(190): e64681 doi: 10.3791/64681 [7] LEV I, BRIL R, LIU Y N, et al. Inter-generational consequences for growing Caenorhabditis elegans in liquid[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2019, 374(1770): 20180125 doi: 10.1098/rstb.2018.0125 [8] SZEWCZYK N J, KOZAK E, CONLEY C A. Chemically defined medium and Caenorhabditis elegans[J]. BMC Biotechnology, 2003, 3(1): 19 doi: 10.1186/1472-6750-3-19 [9] 张普, 王巍, 卢盈宇, 等. 线虫液体培养和监测过程关键参数的实验研究[J]. 载人航天, 2020, 26(3): 284-290 doi: 10.3969/j.issn.1674-5825.2020.03.003ZHANG Pu, WANG Wei, LU Yingyu, et al. Study on key parameters of Caenorhabditis elegans liquid culture and monitoring[J]. Manned Spaceflight, 2020, 26(3): 284-290 doi: 10.3969/j.issn.1674-5825.2020.03.003 [10] SCOTT A, WILLIS C R G, MURATANI M, et al. Caenorhabditis elegans in microgravity: an omics perspective[J]. iScience, 2023, 26(7): 107189 [11] SONI P, ANUPOM T, LESANPEZESHKI L, et al. Microfluidics-integrated spaceflight hardware for measuring muscle strength of Caenorhabditis elegans on the International Space Station[J]. NPJ Microgravity, 2022, 8(1): 50 doi: 10.1038/s41526-022-00241-4 [12] NELSON G A, SCHUBERT W W, KAZARIANS G A, et al. Radiation effects in nematodes: results from IML-1 experiments[J]. Advances in Space Research, 1994, 14(10): 87-91 doi: 10.1016/0273-1177(94)90455-3 [13] GAO Y, XU D, ZHAO L, et al. The DNA damage response of C. elegans affected by gravity sensing and radiosensitivity during the Shenzhou-8 spaceflight[J]. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2017, 795: 15-26 doi: 10.1016/j.mrfmmm.2017.01.001 [14] QIAO L, LUO S, LIU Y D, et al. Reproductive and locomotory capacities of Caenorhabditis elegans were not affected by simulated variable gravities and spaceflight during the Shenzhou-8 mission[J]. Astrobiology, 2013, 13(7): 617-625 doi: 10.1089/ast.2012.0962 [15] 罗雅婧. 空间微重力对线虫肌肉运动调控机制的分析[D]. 大连: 大连海事大学, 2018LUO Yajing. Regulatory Mechanism of Space Microgravity on Muscle Movement of C. Elegans[D]. Dalian: Dalian Maritime University, 2018 [16] YANG Q Q, ZHONG R T, CHANG W B, et al. WormSpace μ-TAS enabling automated on-chip multi-strain culturing and multi-function imaging of Caenorhabditis elegans at the single-worm level on the China Space Station[J]. Lab on a Chip, 2024, 24(14): 3388-3402 doi: 10.1039/D4LC00210E [17] HIGASHIBATA A, SZEWCZYK N J, CONLEY C A, et al. Decreased expression of myogenic transcription factors and myosin heavy chains in Caenorhabditis elegans muscles developed during spaceflight[J]. Journal of Experimental Biology, 2006, 209(16): 3209-3218 doi: 10.1242/jeb.02365 [18] HIGASHITANI A, HIGASHIBATA A, SASAGAWA Y, et al. Checkpoint and physiological apoptosis in germ cells proceeds normally in spaceflown Caenorhabditis elegans[J]. Apoptosis, 2005, 10(5): 949-954 doi: 10.1007/s10495-005-1323-3 [19] HARADA S, HASHIZUME T, NEMOTO K, et al. Fluid dynamics alter Caenorhabditis elegans body length via TGF-β/DBL-1 neuromuscular signaling[J]. npj Microgravity, 2016, 2(1): 16006 doi: 10.1038/npjmgrav.2016.6 [20] THEN S M, JUSOH N F, HARUN R, et al. Multi-generational culture of C. elegans on a long-term space flight revealed changes in expression of genes involved in longevity, DNA repair, and locomotion[J]. Asia-Pacific Journal of Molecular Medicine, 2014, 4(2): 1 [21] HARTMAN P S, HLAVACEK A, WILDE H, et al. A comparison of mutations induced by accelerated iron particles versus those induced by low earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans[J]. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2001, 474(1/2): 47-55 doi: 10.1016/s0027-5107(00)00154-8 [22] PAN P, QIN Z, SUN W, et al. A spiral microfluidic device for rapid sorting, trapping, and long-term live imaging of Caenorhabditis elegans embryos[J]. Microsystems & Nanoengineering, 2023, 9: 45 [23] 张普. 秀丽隐杆线虫空间在轨培养实验方法的研究[D]. 大连: 大连海事大学, 2020ZHANG Pu. The Study of Experimental Method for C. elegans Space in-Orbit Culture[D]. Dalian: Dalian Maritime University, 2020 [24] 王巍, 元姝棋, 邱辉, 等. 线虫在轨液体培养荧光观测关键参数研究[J]. 载人航天, 2022, 28(5): 637-645 doi: 10.3969/j.issn.1674-5825.2022.05.010WANG Wei, YUAN Shuqi, QIU Hui, et al. Research on key parameters of fluorescence observation in Caenorhabditis Elegans on-orbit liquid cultivation[J]. Manned Spaceflight, 2022, 28(5): 637-645 doi: 10.3969/j.issn.1674-5825.2022.05.010 [25] 卢盈宇. 空间站荧光标记线虫在轨培养及监测方法建立[D]. 大连: 大连海事大学, 2021LU Yingyu. Establishment of On-orbit Cultivation and Monitoring Methods for Fluorescently Labeled Nematodes in Space Station[D]. Dalian: Dalian Maritime University, 2021 [26] WIGHTMAN B, CORSI A K, CHALFIE M. A transparent window into biology: a primer on Caenorhabditis elegans[J]. Genetics, 2015, 200(2): 387-407 doi: 10.1534/genetics.115.176099 [27] YU Y J, HUA X, CHEN H B, et al. Tetrachlorobisphenol a mediates reproductive toxicity in Caenorhabditis elegans via DNA damage-induced apoptosis[J]. Chemosphere, 2022, 300: 134588 doi: 10.1016/j.chemosphere.2022.134588 [28] LI C Y, WANG Z, SONG B B, et al. Arsenolipid-induced reproductive toxicity in Caenorhabditis elegans: elucidating the mechanism through the HUS-1-CEP-1-EGL-1-CED-9-CED-4-CED-3 signaling pathway[J]. Food and Chemical Toxicology, 2025, 200: 115340 doi: 10.1016/j.fct.2025.115340 [29] HUA X, FENG X, HUA Y S, et al. Paeoniflorin attenuates polystyrene nanoparticle-induced reduction in reproductive capacity and increase in germline apoptosis through suppressing DNA damage checkpoints in Caenorhabditis elegans[J]. Science of the Total Environment, 2023, 871: 162189 doi: 10.1016/j.scitotenv.2023.162189 [30] LIU Z Y, BIAN Q, WANG D Y. Exposure to 6-PPD quinone causes ferroptosis activation associated with induction of reproductive toxicity in Caenorhabditis elegans[J]. Journal of Hazardous Materials, 2024, 471: 134356 doi: 10.1016/j.jhazmat.2024.134356 [31] ODIBA A S, EZECHUKWU C S, LIAO G Y, et al. SMC-5/6 complex subunit NSE-1 plays a crucial role in meiosis and DNA repair in Caenorhabditis elegans[J]. DNA Repair, 2024, 137: 103669 doi: 10.1016/j.dnarep.2024.103669 [32] GODTHI A, MIN S, DAS S, et al. Neuronal IL-17 controls Caenorhabditis elegans developmental diapause through CEP-1/p53[J]. Proceedings of the National Academy of Sciences of the United States of America, 2024, 121(12): e2315248121 doi: 10.1101/2022.11.22.517560 [33] SHAO Y T, LI Y H, WANG D Y. Polylactic acid microplastics cause transgenerational reproductive toxicity associated with activation of insulin and hedgehog ligands in C. elegans[J]. Science of the Total Environment, 2024, 942: 173746 doi: 10.1016/j.scitotenv.2024.173746 [34] REZA R N, SERRA N D, DETWILER A C, et al. Noncanonical necrosis in 2 different cell types in a Caenorhabditis elegans NAD+ salvage pathway mutant[J]. G3 Genes Genomes Genetics, 2022, 12(4): jkac033 doi: 10.1093/g3journal/jkac033 [35] WU Z, CARDONA E A, COHN J A, et al. Nonapoptotic role of EGL-1 in exopher production and neuronal health in Caenorhabditis elegans[J]. Proceedings of the National Academy of Sciences of the United States of America, 2025, 122(2): e2407909122 -
-





 元姝棋 女, 1996年10月出生于天津市, 大连海事大学环境科学与工程学院环境科学与工程专业博士生. 主要研究方向为空间辐射生物学, 具体研究内容为空间辐射对线虫DNA损伤修复的作用机制. E-mail:
元姝棋 女, 1996年10月出生于天津市, 大连海事大学环境科学与工程学院环境科学与工程专业博士生. 主要研究方向为空间辐射生物学, 具体研究内容为空间辐射对线虫DNA损伤修复的作用机制. E-mail: 

 下载:
下载: